Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Product Highlights
- Clinically proven relief from vaginal discomfort
- Specific probiotic strains naturally originate from humans
- Strain patents in China, Taiwan and USA
- Strain deposit: AP-32 at CCTCC (M2011127) and BCRC (910437); F-1 at BCRC (910469); CT-53 at BCRC (910468); TE-33 at BCRC (910441)
- Product Background
- Female consumer power surpasses males, driving the demand for women's health products.
- Intimate health, a widely discussed topic in the healthcare industry, is particularly significant due to high treatment costs, recurrence rates, and low cure rates.
- Common issues include bacterial vaginosis, trichomoniasis, and candidiasis, leading to genital itching and discomfort.
- Younger consumers seek long-term maintenance and care, shifting the focus from treating diseases to empowering women's health.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Use Level
- 10¹⁰ CFU
- Product Applications
- Functional foods and dietary supplements in forms of capsules, tablets, powdered sachets, and others
- Vaginal health: relieve vaginal discomfort
- Application Information
- R&D Center introduces PRONULIFE® VagProtect, a patented anti-inflammatory strain available in the US, Taiwan, and China.
- It effectively restores the vaginal microbiota, even when used alongside antibiotics for vaginal infections.
- With sustained high activity up to the intestines, human trials validate its efficacy in addressing various symptoms related to vaginal infections.
Properties
- Specifications
Value Units Test Method / Conditions Specification 10¹¹ cfu/g - - Contents
- Lactobacillus salivarius AP-32
- Lactobacillus rhamnosus F-1
- Lactobacillus rhamnosus CT-53
- Lactobacillus reuteri TE-33
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
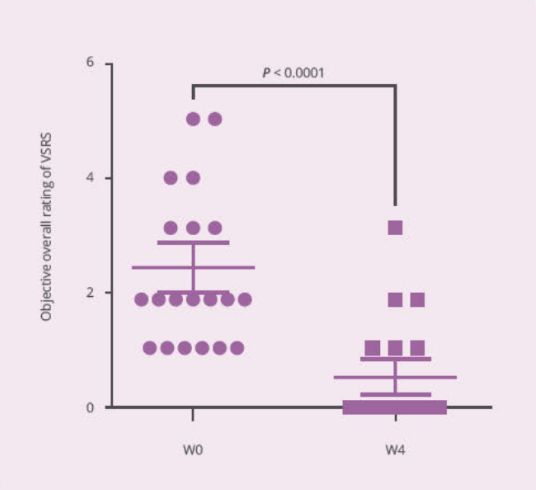
- Clinical Study
Clinical Study on 32 subjects for 4 weeks, with 12 healthy subjects and 20 subjects with vaginal discomfort (administered capsules containing PRONULIFE® VagProtect and rated with Vaginitis Symptom Rating Scale [VSRS])
- PRONULIFE® VagProtect significantly improves specific vaginal discomfort, such as secretions, odors, and itching and burning sensation
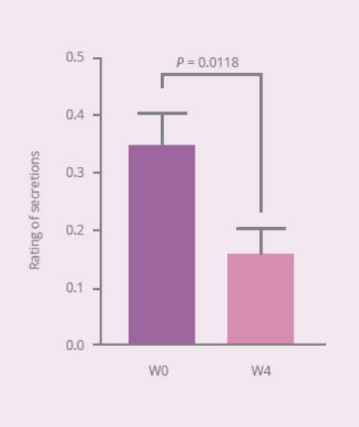
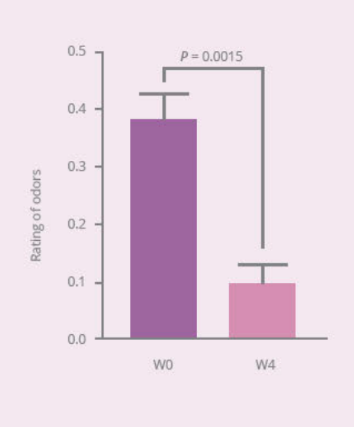
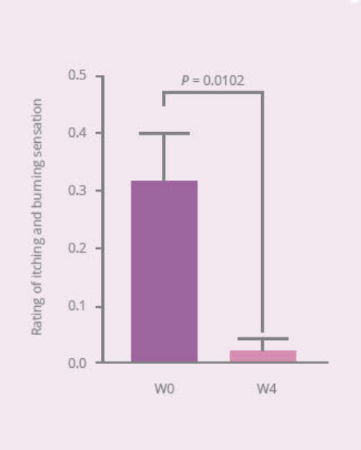
- PRONULIFE® VagProtect significantly improves subjective and objective overall vaginal discomfort
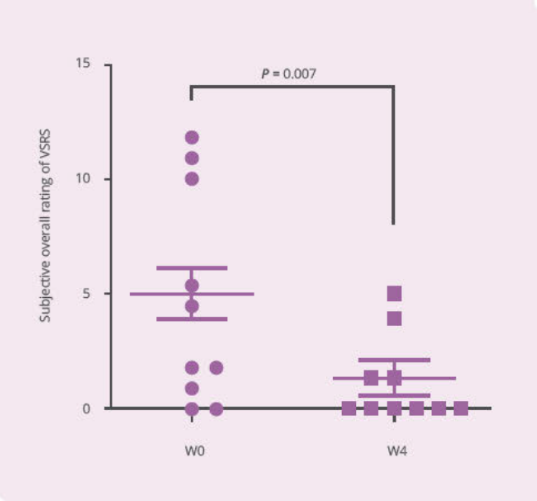
Storage & Handling
- Shelf Life
- 2 years
- Storage and Shelf Life Conditions
- Shelf life: 2 years
- Storage: -16 to 20°C

