Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
- C₂₀H₂₂N₈O₅
- Technologies
- Product Families
- Chemical Structure
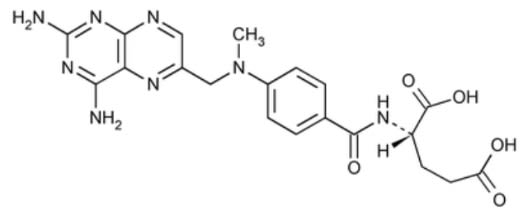
- Defination
Methotrexate contains NLT 98.0% and NMT 102.0% of methotrexate (C₂₀H₂₂N₈O₅), calculated on the anhydrous basis.
Applications & Uses
Properties
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 454.45 - -
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP R-Methotrexate RS
- (R)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl) amino}benzamido)pentanedioic acid.
- C₂₀H₂₂N₈O₅ 454.45
- Sodium (R)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl] (methyl)amino}benzamido)pentanedioate.
- C₂₀H₂₀N₈Na₂O₅ 498.41
- USP Methotrexate System Suitability Mixture RS
This mixture contains:
- Methotrexate Methotrexate dimethylester hydrochloride; (S)-Dimethyl-2-(4-{[(2,4-diaminopteridin-6-yl)methyl] (methyl)amino}benzamido) pentanedioate hydrochloride. C₂₂H₂₆N₈O₅
- Methotrexate related compound I; (S)-4-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl) amino}benzamido)-5-methoxy-5-oxopentanoic acid. C₂₁H₂₄N₈O₅ 468.47
- USP Methotrexate RS USP Methotrexate Related Compound B RS (S)-2-{4-[(2,4-Diaminopteridin-6-yl)methylamino] benzamido}pentanedioic acid. C₁₉H₂₀N₈O₅ 440.42 USP
- Methotrexate Related Compound C RS (S)-2-(4-{[(2-Amino-4-oxo-1,4-dihydropteridin-6-yl) methyl](methyl)amino}benzamido)pentanedioic acid. C₂₀H₂₁N₇O₆ 455.42
- USP Methotrexate Related Compound E RS 4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino} benzoic acid, hemihydrochloride. C₁₅H₁₅N₇O₂ · 1⁄2HCl 343.56
Storage & Handling
- Storage Information
Preserve in tight, light-resistant containers.
