Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
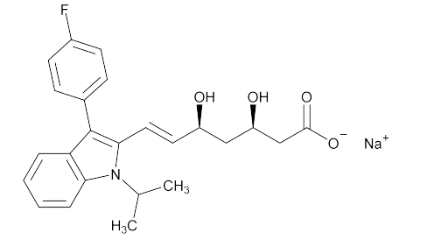
- C₂₄H₂₅FNNaO₄
- Technologies
- Product Families
- Definition
Fluvastatin Sodium contains NLT 98.0% and NMT 102.0% of fluvastatin sodium (C24H25FNNaO4), calculated on the anhydrous basis.
- Chemical Structure
Properties
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 433.45 - -
Regulatory & Compliance
- Certifications & Compliance
- USP Reference Standards
- USP Fluvastatin Sodium RS
- USP Fluvastatin Related Compound B RS
- Fluvastatin t-butyl ester. = [R*,S*-E]-(±)-7-[3-(4-Fluorophenyl)-1-methylethyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid 1,1-dimethylethyl ester; also known as tert-Butyl (3RS, 5SR,E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2- yl]-3,5-dihydroxyhept-6-enoate. C28H34FNO4 467.58
- USP Fluvastatin for System Suitability RS
- Fluvastatin sodium, containing 1%–2% of the fluvastatin sodium anti-isomer.
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Storage & Handling
- Storage Information
Preserve in tight, light-resistant containers, protected from moisture. Store at a temperature not exceeding 40°.
