Enhanced TDS
Identification & Functionality
- Active Component
- Country of Origin
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Molecular formula
- C₁₅H₁₀O₅
- Technologies
- Product Families
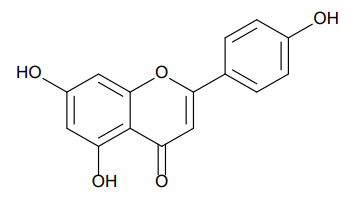
- Chemical Structure
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
Properties
- Physical Form
- Soluble In
- Appearance
- Light yellow fine powder
- Odor
- Characteristic
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 270.24 - - - Active Ingredient
Value Units Test Method / Conditions Purity min. 98.0 % HPLC - Microbiological Values
Value Units Test Method / Conditions Total Plate Count max. 1000 CFU/g Ch.P.C.Rule 80 Yeasts & Molds Count max. 100 CFU/g Ch.P.C.Rule 80 Salmonella Negative - Ch.P.C.Rule 80 Escherichia coli Negative - Ch.P.C.Rule 80 - Specifications
Value Units Test Method / Conditions Loss on Drying max. 5.00 % Ch.P.C.Rule 52 Partical Size (pass 80 mesh) 95 % Ch.P.C.Rule 47 - Heavy Metals
Value Units Test Method / Conditions Heavy Metals max. 10 ppm Ch.P.C.Rule 21 Arsenic Content max. 2 ppm Ch.P.C.Rule 21 Lead Content max. 2 ppm Ch.P.C.Rule 21 Mercury Content max. 0.5 ppm Ch.P.C.Rule 21
Regulatory & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Packaging & Availability
Storage & Handling
- Shelf Life
- 24 months
- Storage Conditions
- Keep in sealed and away from light; Cold, drying.
- Keep for 24 months under the above conditions.
