Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
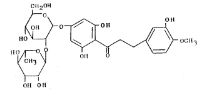
- Molecular formula
- C₂₈H₃₆O₁₅
- Technologies
- Product Families
- Molecular Formula
Features & Benefits
Properties
- Physical Form
- Appearance
- White or yellowish-white powder
- Soluble in
- Dimethyl Sulfoxide, Cool Water is 0.4-0.5 g/l but is very Soluble in Hot Water
- Insoluble in
- Methylene Chloride, Water
- Typical Properties
Value Units Test Method / Conditions Water Content** max. 12 % Current EP Sulphated Ash max. 0.2 % Current EP - Microbiological Values
Value Units Test Method / Conditions Aerobic Count max. 1000 cfu/g - Coliform Count Absent /g - Yeast and Mold Count max. 100 cfu/g - - Assay
Value Units Test Method / Conditions Assay 96.0 - 101.0 % Current EP - Related Substances
Value Units Test Method / Conditions Impurity B (neodiosmin) max. 2 - - Impurity D (naringin-dihydrochalcone) max. 2 - - Other Impurity max. 0.5 - - Total Impurities Other Than Impurity B max. 2.5 - - - Heavy Metals
Value Units Test Method / Conditions Heavy Metals max. 10 ppm Current EP - Note
**NHP-DC crystallizes with 4 molecules of water forming a tetrahydrate. This corresponds to 10.5% water content.
- Stability
NHDC is very stable in crystalline form and in water solutions making it particularly suitable as a sweetener for beverages. It withstands high temperatures, and retains its characteristics following pasteurisation or sterilisation processes.
Regulatory & Compliance
Packaging & Availability
Storage & Handling
- Shelf Life
- 2 years
- Storage and Handling Conditions
NHDC is very stable in crystalline form and in water solutions making it particularly suitable as a sweetener for beverages. It withstands high temperatures, and retains its characteristics following pasteurization or sterilization processes.
- Shelf Life Conditions
Two years when stored as above.
