Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Properties
- Physical Form
- Appearance
- White to light brown color powder
- Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 100 cfu/g - Staphylococcus Aureus Negative cfu/g - Salmonella Negative cfu/g - Listeria Monocytogenes Negative cfu/g - Eschericha Coli Negative cfu/g - Coliforms Negative cfu/g - - Specifications
Value Units Test Method / Conditions Water Activity max. 0.25 aw - Viable Cell Counts min. 3.0× 10¹¹ cfu/g - Moisture Content max. 7 % - - Composition
NO. Composition Content (%) 1 Lactobacillus fermentum TSF331 100% Total 100%
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Product Characteristics
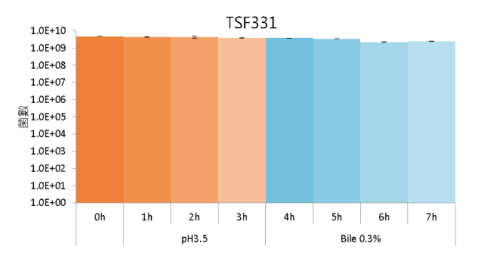
High Gastric Acid and Bile Tolerance:
TSF331 remained high bioavailability in the simulated environment with Gastric Acid and Bile for 7 hours.
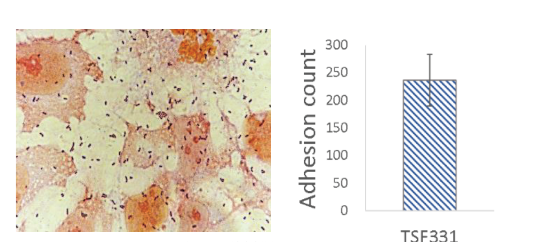
Strong Colonization on Caco-2 Intestine Cells:
TSF331 highly adheres to Caco-2 intestine cells
Uric acid level reduction:
Human trial: 53 subjects with high uric acid level taking probiotics 3 times per day for 60 days
TSF331 can effectively reduce uric acid level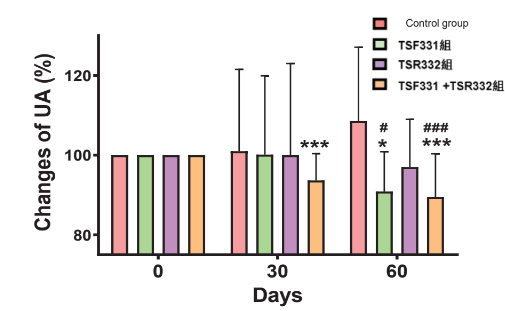
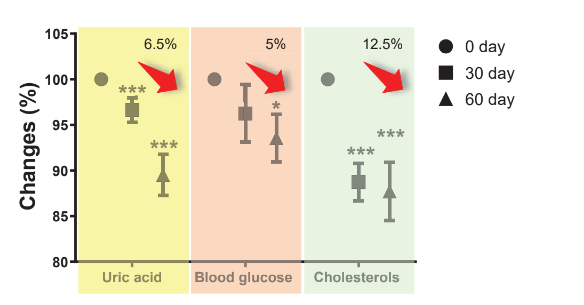
Blood glucose and lipids regulation:
Human trial: 25 subjects with high uric acid level taking probiotics 3 times per day.
Packaging & Availability
- Packaging Type
- Packaging Information
1kg a bag with nitrogen filling and vacuum packing.
Storage & Handling
- Shelf Life
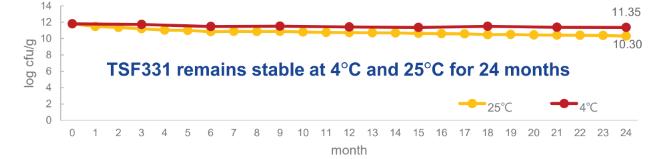
- 24 months
- Stability Test
TSF331 remains stable at 4°C and 25°C for 24 months:
TSF331 remains stable at -20°C for 36 months:


